Why Do We Need Clinical Trials in Pediatric Oncology?
Clinical trials, often referred to as "clinical studies" or simply "studies," are essential for the development of new therapies and medications. Their purpose is to prove the effectiveness of new drugs and to ensure their safety and tolerability.
In pediatric oncology, clinical trials focus on improving the overall treatment of critically ill children and adolescents while providing them access to innovative therapies. This is crucial for developing medications that are specifically safe and effective for children, as children are not just small adults—they have unique physiological needs.
Through our research, we aim to continuously optimize treatments for severely ill children, increase survival rates for many young patients, and reduce treatment side effects.
However, such clinical studies must meet strict medical and legal requirements, especially when children are involved. Ensuring patient safety and the ethical justification of administering medications is always the top priority.
AML-BFM Study Group
Learn moreOur Work at a Glance
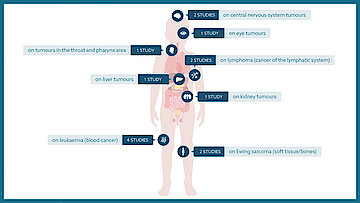
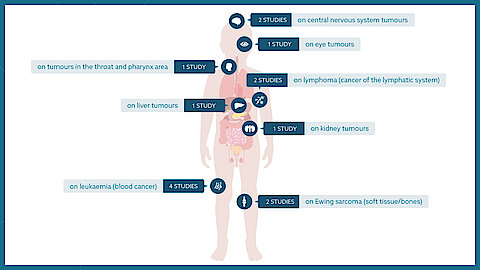
To ensure that as many children and adolescents as possible have access to clinical trials, these studies are conducted not only on a national level but also internationally.
National and international partners
International partnerships are essential for this effort. In Germany, the PRN works closely with the German Society for Pediatric Oncology and Hematology (GPOH) and pediatric and adolescent medicine clinics across the country. Additionally, it collaborates with European research groups such as the Princess Máxima Center (PMC) and the Italian Association for Pediatric Hematology and Oncology (AIEOP).
Across the ocean, the PRN maintains close cooperation with some of the world's largest nonprofit organizations in pediatric hematology and oncology, including The Leukemia & Lymphoma Society (LLS), the Children's Oncology Group (COG), and the National Cancer Institute (NCI).
This collaboration aims to reduce the number of competing clinical trials, accelerate progress by bringing together patients, hospitals, and study groups, and centralizing and enhancing the quality of study results.

